There remain gaps in our knowledge of hereditary and sporadic causes of hematological malignancy (HM) and bone marrow failure (BMF) that prevent optimal diagnosis, disease surveillance and treatment. Here we report the discovery of ERG as a novel predisposition gene for BMF and HM. ERG is a known oncogene, typically via gene-fusions, leading to dysregulated ERG overexpression in blood and solid cancers. We identified a germline ERG ETS domain variant p.Y373C segregating with thrombocytopenia in a mother, who progressed to AML (27 yr) and then therapy-related MDS (35 yr), and in her 2 sons. All three showed copy neutral loss of heterozygosity of all or part of chromosome 21q, including the ERG locus, with the oldest son showing at least 2 somatic genetic rescue (SGR) events. The possibility of causal RUNX1 variants were ruled out, with the smallest somatic cnLOH event beginning within the RUNX1 gene, but not encompassing the RUNT domain where the majority of pathogenic missense variants are located. ERG, a highly constrained gene (LOEUF <0.33), is critical for definitive hematopoiesis, adult hematopoietic stem cell (HSC) function and platelet maintenance. An identical corresponding heterozygous germline variant (p.Y343C) in ERG’s closest gene by homology, FLI1, causes platelet-type bleeding disorder-21 (BDPLT21, OMIM #617443).
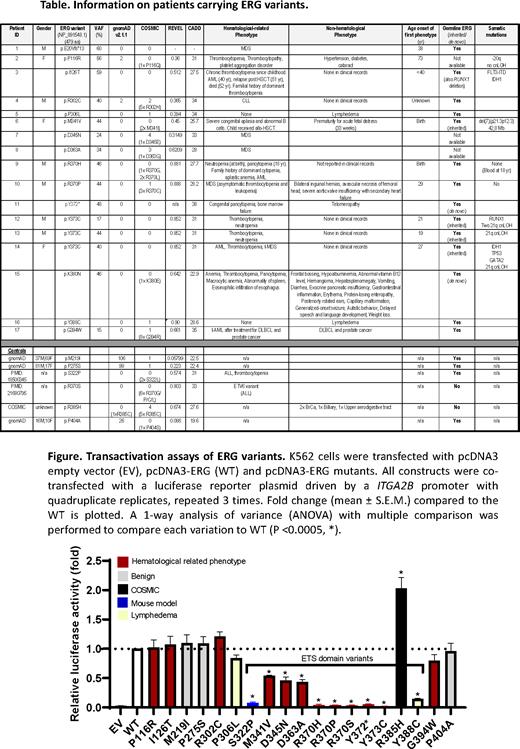
Through global collaborations, we have identified 15 heterozygous variants in the ERG gene, 13 of which are missense and 2 truncating variants, in 17 individuals with cytopenia and/or HM (mainly myeloid) or lymphedema (Table). Onset of hematological symptoms ranged from birth to 38 years for truncating and constrained ETS domain variants. Of these 15 variants, 12 have been confirmed germline including 2 de novo. Only 4 meiotic transmissions are observed. None of the missense variants in the highly conserved ETS domain of ERG which mediates DNA binding, protein-protein interactions and nuclear localization, are present in gnomAD. We have functionally characterized 19 ERG variants, 12 potentially pathogenic, 1 known mouse pathogenic variant and 3 population controls demonstrating that most ETS domain missense variants display loss-of-function (LOF) characteristics disrupting transcriptional transactivation (Figure), DNA-binding and/or nuclear localization in vitro. Robust preliminary data from ex vivo models of ERG overexpression in mouse fetal liver cells in tissue culture (cytokine-independence), a mouse transplant assay and previous germline mutant Erg mouse models are concordant with ETS domain missense variants being LOF compared to wildtype ERG and benign controls. Together, these data provide clinical, in vitro and ex vivo functional studies implicating LOF variants in hematological disease predisposition. LOF ERG mutations also occur in sporadic cases of HM.
Recently, as part of a Genomics England Research Consortium population study, 4 truncating ERG variants were described in 7 individuals across 4 families with 3 meiotic transmissions and a de novo case with primary lymphedema (1) and we add 2 novel missense variants here. One patient showed SGR across the ERG locus in blood. Blood phenotypes were not described. Our results demonstrate that germline ERG variants predispose to diverse cytopenia, BMF and HM in both children and adults. In our family mentioned above, the mother received an unrelated alloHSCT due to t-MDS while her 2 sons with cytopenias continue to be monitored. The natural history of this new syndrome will require careful identification of germline lesions with additional longitudinal studies in more patients and families needed. This ERG syndrome parallels GATA2 deficiency syndrome (HM and lymphedema) and RUNX1 Familial Platelet disorder-myeloid malignancy (thrombocytopenia and HM). Like the well-known disease genes GATA2 and RUNX1, ERG is also a member of the transcription factor heptad involved in HSC maintenance and differentiation. ERG adds to a growing list of genes whose unregulated expression contributes to HM and other cancers.
Identification of causal germline ERG variants like those outlined in this study, has direct clinical implications for patient and family management including diagnosis, counselling, surveillance and treatment strategies such as selection of bone marrow transplant donors and potential for targeted therapies including gene and cell therapy.
Reference
1. Greene D et al. Nat.Med. 29:679-688 2023
Disclosures
Scott:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sicre De Fontbrune:Alexion, AstraZeneca Rare Disease: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Sobi: Honoraria, Research Funding; Samsung: Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Mutsaers:AstraZeneca: Research Funding; GlaxoSmithKline: Consultancy. DeMille:ARUP Laboratories: Current Employment. Crawford:Illumina: Current Employment. Soulier:STRM.Bio: Membership on an entity's Board of Directors or advisory committees; Rocket Pharmaceuticals: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal